Transient protein encounters characterized by paramagnetic NMR†
Abstract
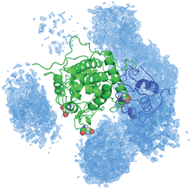
Invisible to most biophysical techniques, transient intermediates formed during biomolecular association orchestrate protein recognition and binding. Here, we study such minor species mediating the interaction between physiological partners, cytochrome c and cytochrome c peroxidase, by paramagnetic relaxation enhancement NMR spectroscopy. The visualization of multiple protein–protein orientations constituting the transient encounter state reveals a broad spatial distribution, which is in striking agreement with that obtained in earlier theoretical simulations. Being inactive in the intermolecular electron transfer, the encounter complex pre-orients the interacting molecules, enabling a reduced dimensionality search of the dominant, functionally active bound form. The encounter complex is insensitive to the redox and spin states of the interacting molecules, suggesting that its properties are determined by protein polypeptides rather than heme cofactors.

 Please wait while we load your content...
Please wait while we load your content...