Regioselective and diastereoselective aminoarylation of 1,3-dienes†
Abstract
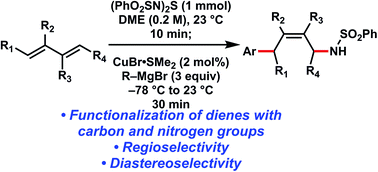
The 1,4-functionalization of dienes is a synthetically useful strategy for incorporating molecular complexity into a class of simple substrates. We report the aminoarylation of acyclic and cyclic 1,3-dienes via the sequential [4 + 2] cycloaddition with a sulfurdiimide reagent and copper-catalyzed allylic substitution with Grignard reagents. The regioselective and diastereoselective aminoarylation of unsymmetrical dienes is also presented, which highlights the utility of this method for generating products with multiple functional groups and stereocenters.

 Please wait while we load your content...
Please wait while we load your content...