Topotactic structural conversion and hydration-dependent thermal expansion in robust LnMIII(CN)6·nH2O and flexible ALnFeII(CN)6·nH2O frameworks (A = Li, Na, K; Ln = La–Lu, Y; M = Co, Fe; 0 ≤ n ≤ 5)†
Abstract
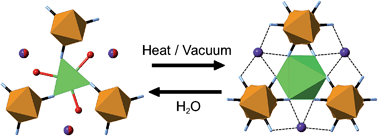
The structures of the AxLnM(CN)6·nH2O (A = Li, Na, K; Ln = La–Lu, Y; M = Co, Fe; x = 0, 1; 0 ≤ n ≤ 5) cyanide frameworks, their thermal expansion behaviour, and their transformations upon dehydration are explored using X-ray and neutron single crystal diffraction and X-ray powder diffraction. Modification from positive to negative thermal expansion in the LnCo(CN)6·nH2O phases is achieved by removal of the guest water molecules. Most notable is the unprecedented flexibility demonstrated by the “coiling” of KLnFe(CN)6·nH2O frameworks upon their dehydration, wherein the lanthanoid coordination geometry reversibly converts from a 9-coordinate tri-capped trigonal prism to a 6-coordinate octahedron via a single-crystal-to-single-crystal process, accompanied by a large (14–16%) decrease in unit cell volume.

 Please wait while we load your content...
Please wait while we load your content...