Selective radical amination of aldehydic C(sp2)–H bonds with fluoroaryl azides via Co(ii)-based metalloradical catalysis: synthesis of N-fluoroaryl amides from aldehydes under neutral and nonoxidative conditions†
Abstract
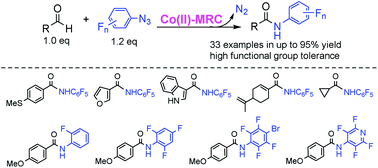
The Co(II) complex of the D2h-symmetric amidoporphyrin 3,5-DitBu-IbuPhyrin, [Co(P1)], has proven to be an effective metalloradical catalyst for intermolecular amination of C(sp2)–H bonds of aldehydes with fluoroaryl azides. The [Co(P1)]-catalyzed process can employ aldehydes as the limiting reagents and operate under neutral and nonoxidative conditions, generating nitrogen gas as the only byproduct. The metalloradical aldehydic C–H amination is suitable for different combinations of aldehydes and fluoroaryl azides, producing the corresponding N-fluoroaryl amides in good to excellent yields. A series of mechanistic studies support a stepwise radical mechanism for the Co(II)-catalyzed intermolecular C–H amination.

 Please wait while we load your content...
Please wait while we load your content...