A tripodal peptide ligand for asymmetric Rh(ii) catalysis highlights unique features of on-bead catalyst development†
Abstract
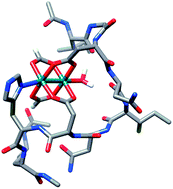
A tridentate eq-eq-ax peptide ligand for rhodium(II) complexes, discovered by high-throughput on-bead screening, is an efficient and selective catalyst for asymmetric cyclopropanation reactions. The metallopeptide catalyst is easily prepared and screened on bead. The axial imidazole ligand significantly improves catalyst function over previous mono-peptide Rh(II) catalysts. Experimental and theoretical investigations shed light on the role of the imidazole ligand and the structural consequences of imidazole ligation. These studies also explain the differential behavior of these metallopeptide catalysts in solution and on bead, and provide insight into the development of immobilized homogeneous catalysts in general.

 Please wait while we load your content...
Please wait while we load your content...