Synthesis and properties of fully-conjugated indacenedithiophenes†
Abstract
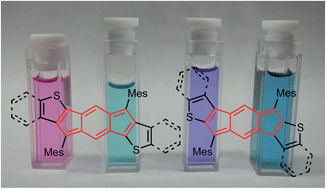
The synthesis and characterization of four fully-conjugated indacenedithiophenes (IDTs) are disclosed. In contrast to anthradithiophenes, regioselective synthesis of both syn and anti isomers is readily achieved. Thiophene fusion imparts increased paratropic character on the central indacene core as predicted by DFT calculations and confirmed by 1H NMR spectroscopy. IDTs exhibit red-shifted absorbance maxima with respect to their all-carbon analogues and undergo two-electron reduction and one-electron oxidation.

 Please wait while we load your content...
Please wait while we load your content...