Structural studies of adsorbed protein (betalactoglobulin) on natural clay (montmorillonite)
Abstract
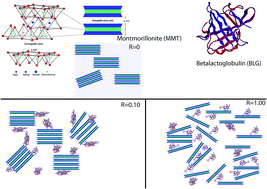
In this work, the adsorption of a small globular protein (betalactoglobulin, BLG), on a natural montmorillonite clay (Mt) was investigated in acidic buffer (pH = 3). The combination of different characterization techniques such as zetametry, X-ray diffraction, transmission electronic microscopy, fluorescence and solid state nuclear magnetic resonance spectroscopies shed light on the interaction mechanism between the clay mineral and the proteins. For low BLG concentration, a slight increase of the interlayer spacing of the clay mineral was noticed as well as structural changes of the protein. In contrast, as the concentration of BLG increased, the adsorption led to a partial exfoliation of the clay mineral, accompanied with significant secondary structural changes of the protein characterized by a loss of β-sheet organization. Altogether, our results revealed an unexpected adsorption scheme where the increase of the BLG/Mt weight ratio of the hybrid material leads to a partial exfoliation of the Mt, but at the expense of the protein native structure.

 Please wait while we load your content...
Please wait while we load your content...