Cinchonidine thiourea catalyzed asymmetric addition of phenols to oxindole derivatives†
Abstract
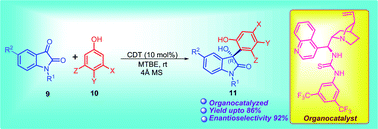
A highly enantioselective Friedel–Crafts reaction of activated phenols with isatin derivatives has been developed employing Cinchona-derived thiourea as an organocatalyst. A variety of biologically important 3-aryl-3-hydroxy-2-oxindoles have been synthesized using phenols in good to excellent yield with good enantioselectivity (up to 92% ee).

 Please wait while we load your content...
Please wait while we load your content...