Structure – chiroptical properties relationship of cisoid enones with an α-methylenecyclopentanone unit†
Abstract
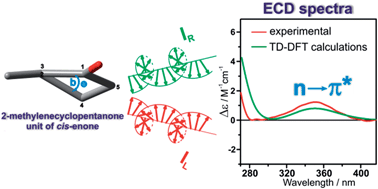
In the present work, the validity of sector and helicity rules correlating the stereostructure of cis-enones containing the 2-methylenecyclopentanone unit with the sign of the nπ* Cotton effect (CE) observed in their electronic circular dichroism (ECD) spectra is assessed. To this end, a series of model steroid cis-enones with five-membered ketone rings was synthesized. To investigate the scope and limitations of existing rules a combination of ECD spectroscopy, X-ray analysis, and time-dependent density functional theory (TD-DFT) calculations were utilized. A comparison of the experimental ECD spectra with spectra simulated by the TD-DFT calculations gave a reasonable interpretation of the nπ* CE's observed in the 360–335 nm spectral range. The results suggest that the previously articulated rules are not applicable to the investigated compounds. On the basis of comprehensive analysis of collected data, a new rule correlating perfectly the structure of studied enones with the signs of their nπ* CE was proposed. This rule correlates directly the sign of the torsion angle “b” of the cyclopentanone ring of cis-enone with the sign of the nπ* CE.

 Please wait while we load your content...
Please wait while we load your content...