Theoretical studies of the hydration reactions of stabilized Criegee intermediates from the ozonolysis of β-pinene
Abstract
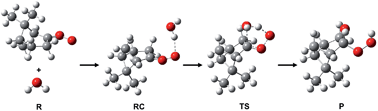
A theoretical study was performed of the reactions of the stabilized Criegee intermediates (sCIs) of β-pinene with H2O and its dimer. Due to the large size of the biogenic sCIs, the transition states of the hydration reactions were explored with the Monte Carlo Transition State Search Program (MCTSSP), which integrated the Monte Carlo sampling technique with a transition state optimization method. The computations were performed with the M06-2X/6-311+G(2d,p) and B3LYP/6-311+G(2d,p) levels of theory. The relative energies showed that the results of the M06-2X functional are in good agreement with the results of the DF-MP2 and CCSD(T) methods. Both the reactions of the β-pinene-sCI with H2O and the β-pinene-sCI with (H2O)2 were found to be strongly exothermic. Activation barrier calculations indicate that the sink reaction with the water dimer may proceed significantly faster than the reaction with the water monomer despite the low concentration of water dimers in the atmosphere. Therefore, the reaction of sCIs with water vapor that includes large water clusters rather than single water molecules should be studied.

 Please wait while we load your content...
Please wait while we load your content...