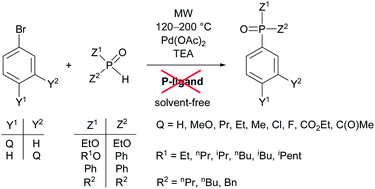
A “green” variation of the Hirao reaction: the P–C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates and secondary phosphine oxides with bromoarenes using a P-ligand-free Pd(OAc)2 catalyst under microwave and solvent-free conditions†
Abstract
The P–C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates, diphenylphosphine oxide and dialkylphosphine oxides with bromoarenes may be performed in the presence of a P-ligand-free Pd(OAc)2 catalyst and triethylamine under microwave-assisted (MW) and, in almost all cases, solvent-free conditions to afford diethyl arylphosphonates, alkyl diphenylphosphinates, aryldiphenylphosphine oxides and dialkylphenylphosphine oxides, respectively. This is the “greenest” accomplishment of the well-known Hirao reaction that has now been found to have general application for a broad spectrum of >P(O)H species with different reactivity and a great variety of substituted bromobenzenes. The alkyl phenyl-H-phosphinates were prepared by the MW-promoted alkylation of phenyl-H-phosphinic acid in the absence of any solvent.

 Please wait while we load your content...
Please wait while we load your content...