Structural optimization of super-gelators derived from naturally-occurring mannose and their morphological diversity†
Abstract
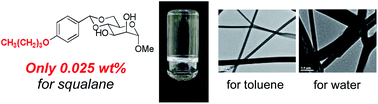
Mannose derivatives were synthesized as low molecular-weight gelators with various alkoxy substituents on the aromatic ring of methyl-4,6-O-benzilidene-α-D-mannopyranoside . Most of these mannose derivatives could gel in various solvents, such as octane, cyclohexane, toluene, ethylene glycol and ethanol solutions, at concentrations lower than 2.0 wt%. In particular, the critical gelation concentration (CGC) of methyl-4,6-O-(4-butoxybenzylidene)-α-D-mannopyranoside (2) for squalane was only 0.025 wt%, one of the lowest CGCs we have ever experienced. The observations of xerogels by FE-SEM, TEM and AFM revealed that the length of the alkoxy chains of the mannose derivatives influences the gel morphologies. Moreover, the toluene gels formed from the mannose derivatives 1–6 functionalized by a linear alkoxy group exhibited thixotropic properties. Interestingly, the gels of various solvents formed from methyl-4,6-O-(4-dodecyloxybenzylidene)-α-D-mannopyranoside (6) (with the longest alkoxy chain on the aromatic ring in this paper) exhibited thixotropic properties. Thus, we confirmed that alkoxy groups on the aromatic ring exert noticeable effects on the gelation properties of these mannose derivatives.

 Please wait while we load your content...
Please wait while we load your content...