Direct electron transfer from alcohol dehydrogenase†
Abstract
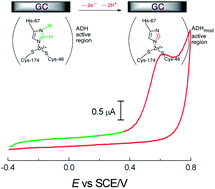
This study employed hydrodynamic cyclic voltammetry (HCV) with a glassy carbon (GC) rotating disk electrode (RDE), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) to investigate the direct electron transfer (DET) behavior of alcohol dehydrogenase (ADH, EC 1.1.1.1) embedded or otherwise in polymyxin (PM) and adsorbed concomitantly with cofactor NAD+ or NADH, or none on GC and covered with Nafion®. The hybrid GC/PM-ADH-NAD+/Nafion electrode thus constructed (and visualized by scanning electron microscopy (SEM)) persistently bioelectrocatalyzed ethanol oxidation and performed DET involving baker's yeast ADH, GC, and ethanol—a key finding in the present study—making this system a promising anode for use in biofuel cells. A rate constant (ks) of 0.82 s−1 was obtained for this electrode at a potential scan rate (ν) of 120 mV s−1. EIS experiments, particularly those conducted after higher potential HCV scans, allowed resistance to electron hopping (Reh) between redox centers in ADH and between these centers and ethanol molecules to be estimated as 84 kΩ lower for the GC/PM-ADH-NAD+/Nafion electrode during ethanol oxidation than for bare GC in the presence of ethanol. Nuclear magnetic resonance (NMR) unequivocally confirmed acetaldehyde production from ethanol oxidation by ADH DET.

 Please wait while we load your content...
Please wait while we load your content...