Regioselective opening of unsymmetrical cyclic anhydrides: synthesis of N-glycosylated isoasparagine and isoglutamine conjugates†
Abstract
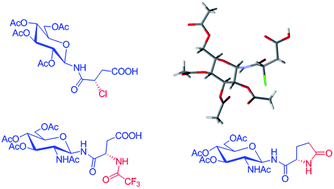
N-Glycopeptide mimetic with N-glycosylated isoasparagine and isoglutamine conjugates were synthesized by regioselective opening of unsymmetrical cyclic anhydride derivatives of L-aspartic acid and L-glutamic acid, using per-O-acetylated β-D-glycopyranosyl amine. The α-chloro derivative gave a mixture of asparagine and isoasparagine linked glycoconjugates, whereas the trifluoroacetamide derivatives gave predominantly the isoasparagine and isoglutamine linked glycoconjugates as the product. The X-ray crystal structure of the α-chloro isoasparagine linked glycoconjugate showed unique pattern of hydrogen bonding.

 Please wait while we load your content...
Please wait while we load your content...