Larger π-extended anti-/syn-aroylenediimidazole polyaromatic compounds: synthesis, physical properties, self-assembly, and quasi-linear conjugation effect†
Abstract
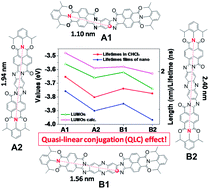
Four π-extended anti-/syn-aroylenediimidazole (ADI) polyaromatic compounds (1, 2, 3, and 4) with 11- or 13-fused rings have been successfully synthesized via a tandem cyclocondensation reaction between tetraamines and naphthalene dicarboxylic monoanhydride monoimide. The observed optical bandgaps for 1–4 are 2.70 (458 nm), 2.34 (529 nm), 2.31 (537 nm), and 2.21 eV (561 nm), respectively, which are in accordance with the calculated bandgaps from DFT calculations for 1–4, which are 2.77, 2.49, 2.29, and 2.21 eV, respectively. Our results indicate that there are obvious anti-/syn- and π-extended effects in these molecules. The cyclic voltammetry (CV) measurements show that all the compounds exhibit quasi-reversible reduction waves. The experimental LUMO levels from CV show an interesting zigzag-curved change (zigzag-shaped curve) in sequence, which matches well with those of the theoretical calculations. Furthermore, the fitted decay lifetimes of 1–4 in CHCl3 are 1.86, 1.32, 1.55, and 1.42 ns, which have the same trend as the above-mentioned zigzag-shaped curve. These trends are believed to be related to the intrinsically effective quasi-linear conjugation (QLC) with a theoretically calculated quasi-linear length of 1.10 nm, 1.94 nm, 1.56 nm, and 2.40 nm, respectively. The successful synthesis and characterization of four soluble π-extended ADI polyaromatic compounds could provide us with more diverse candidates for air-stable organic electronic devices.

 Please wait while we load your content...
Please wait while we load your content...