One-pot production of hydrocarbon oil from poly(3-hydroxybutyrate)†
Abstract
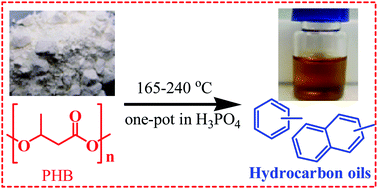
Poly(3-hydroxybutyrate) (PHB) is an energy storage material of many microbial species, and has been found to be an effective feedstock for production of renewable hydrocarbon oils. A high oil yield (up to 38.2 wt%) was obtained in a phosphoric acid (H3PO4) solution at mild temperatures (165–240 °C). PHB and crotonic acid (C4H6O2), a dominant thermal degradation product of PHB, were deoxygenated mainly via decarboxylation, generating similar liquid and gaseous products. Carbon dioxide and propylene were the major products in gas phase with little CO formation. The hydrocarbon oil (C4–C16) is a mixture of alkanes, alkenes, benzenes and naphthalenes. Aromatics (C10–C15) were the major hydrocarbons in a 100 wt% H3PO4 solution, while alkenes and alkanes (C4–C9) were favored in diluted solutions (50 wt% to 85 wt% H3PO4). The concentration of H3PO4 was a key factor that affected the oil composition and yield. A highly efficient decarboxylation of crotonic acid at 220 °C for 3 hours resulted in 70.8 wt% of oxygen being removed as CO2 and 57.0 wt% of carbon being recovered as hydrocarbon oil. The H3PO4 solution can be repeatedly used for high yield oil production. This work shows that a type of new biological feedstock can be used to produce renewable hydrocarbon oil in an efficient one-pot reaction.

 Please wait while we load your content...
Please wait while we load your content...