A porphyrin-linked conjugated microporous polymer with selective carbon dioxide adsorption and heterogeneous organocatalytic performances†
Abstract
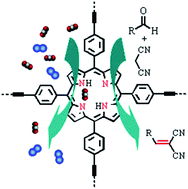
A new porphyrin-based conjugated microporous polymer with rich nitrogen sites in the skeleton has been synthesized by alkyne–alkyne homocoupling reaction. The Brunauer–Emmett–Teller specific surface area up to 662 m2 g−1 was obtained for the new polymer framework with a pore volume of 0.55 cm3 g−1. The polymer network displays high carbon dioxide uptake capacity (up to 3.58 mmol g−1) at 273 K and 1 bar, with good selectivity towards CO2 over N2 and CH4. Furthermore, this framework also acts as a solid organocatalyst towards Knoevenagel reaction of malononitrile with aromatic, heterocyclic aldehydes, and cyclic ketones. The reaction afforded the corresponding products in excellent yields (up to 99%) with short times. Moreover, the heterogeneous catalyst was also found to exhibit an excellent recyclability (up to 10 times) without loss of efficiency.

 Please wait while we load your content...
Please wait while we load your content...