Synthesis of C-spiro-glycoconjugates from sugar lactones via zinc mediated Barbier reaction†
Abstract
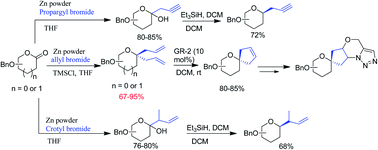
Anomeric gem-diallylation, mono-β-crotylation and mono-β-propargylation of sugar 1,5 and 1,4 lactones have been achieved under Barbier reaction conditions using Zn powder and a catalytic amount of TMSCl as an activator. Ring closing olefin metathesis of the synthesized gem-diallyl derivatives furnished C-spiro cyclopentene glycosides. Finally, the cyclopentene rings were converted into carbohydrate based tricyclic morpholine fused triazole glycoconjugates as potential SGLT2 inhibitors.

 Please wait while we load your content...
Please wait while we load your content...