Hierarchical porous α-Ni(OH)2 grown from a compact ion layer as an electrode by using one-pot synthesis and its pseudocapacitive behaviour†
Abstract
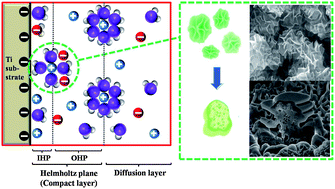
The advancement of supercapacitor electrodes is predicated using simple and efficient synthesis methods. In this study a simple electrochemical precipitation method is presented for the synthesis of an α-nickel hydroxide (α-Ni(OH)2) electrode directly on a Ti substrate without any templates or surfactants. The α-Ni(OH)2 electrode possessed a hierarchically porous structure with well-developed inner and outer pores and a large specific surface area of 205.6 m2 g−1. A supercapacitor with this electrode exhibited a high specific capacitance of 1582 F g−1 in 1 M potassium hydroxide (KOH) at a scan rate of 2 mV s−1 and a capacitive retention of almost 74% after 1000 cycles at a current density of 5 mA cm−2.

 Please wait while we load your content...
Please wait while we load your content...