Direct C–H amination and C–H chloroamination of 7-deazapurines†
Abstract
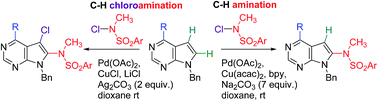
Protocols for selective Pd–Cu-catalyzed direct C–H amination or C–H chloroamination of 7-deazapurines with N-chloro-N-alkyl-arylsulfonamides have been developed leading either to 8-(arylsulfonyl)methylamino-7-deazapurines or to 7-chloro-8-(arylsulfonyl)methylamino-7-deazapurines. The scope and limitations of the methods, as well as synthesis of a small series of 6,8,9-tri- and 6,7,8,9-tetrasubstituted 7-deazapurines and deprotection of the sulfonamide are presented.

 Please wait while we load your content...
Please wait while we load your content...