The role of the ethynylene bond on the optical and electronic properties of diketopyrrolopyrrole copolymers†
Abstract
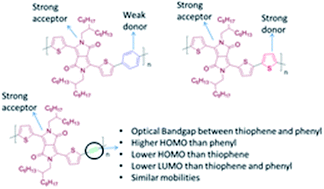
It is presented that the introduction of an alkyne linkage could be an important tool for fine-tuning the electronic and optoelectronic properties of certain donor–acceptor (D–A) conjugated polymers. Extensive optoelectronic studies in an alternating copolymer consisting of diketopyrrolopyrrole (DPP) and ethynylene linkage (TDPPTTB) and comparison over its fully cyclic DPP analogues, such as phenyl (TDPPTP) and thiophene (TDPPTT) rings reveals the role of the ethynylene moiety when it is introduced into the polymer backbone. The ethynylene moiety decreases the donor character of the thiophenes that flank the DPP therefore the highest occupied molecular orbital (HOMO) level of TDPPTTB is situated between the HOMO levels of the TDPPTP and TDPPTT. The optical band gap (Eoptg) of TDPPTTB is fixed between the Eoptg of TDPPTT and TDPPTP with a significant blue-shift in its absorption maximum. Furthermore, detailed studies on the electronic properties of TDPPTTB have been performed in Field Effect Transistors (FETs) using various dielectric materials. Transistors based on TDPPTTB films annealed at 120 °C show ambipolar behaviour, similar to TDPPTP and TDPPTT, with carrier mobilities of 0.03 cm2 V−1 s−1 for holes and 0.02 cm2 V−1 s−1 for electrons.


 Please wait while we load your content...
Please wait while we load your content...