Ring-opening of enantiomerically pure oxa-containing heterocycles with phosphorus nucleophiles†
Abstract
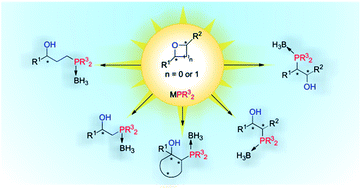
Various oxa-containing heterocycles (i.e. enantiopure epoxide- and oxetane-based substrates) were subjected to ring-opening with phosphorus nucleophiles. The ring-opening reactions proceeded smoothly and the resulting 1,2-, and 1,3-phosphino alcohols were efficiently isolated as stable borane complexes. These derivatives arise from regio- and stereocontrolled syntheses based on ring-opening processes of oxa-containing heterocycles. The regio- and stereochemistry of the resulting chiral products were unequivocally confirmed in many cases via single-crystal X-ray diffraction analysis.

 Please wait while we load your content...
Please wait while we load your content...