New CHARMM force field parameters for dehydrated amino acid residues, the key to lantibiotic molecular dynamics simulations†
Abstract
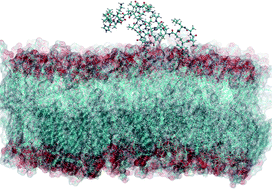
Lantibiotics are an important class of naturally occurring antimicrobial peptides containing unusual dehydrated amino acid residues. In order to enable molecular dynamics simulations of lantibiotics, we have developed empirical force field parameters for dehydroalanine and dehydrobutyrine, which are compatible with the CHARMM all-atom force field. The parameters reproduce the geometries and energy barriers from MP2/6-31G*//MP2/cc-pVTZ quantum chemistry calculations. Experimental, predicted and calculated NMR chemical shifts for the amino protons and α-, β- and carbonyl carbon atoms of the dehydrated residues are consistent with a significant charge redistribution. The new parameters are used to perform the first molecular dynamics simulations of nisin, a widely used but poorly understood lantibiotic, in an aqueous environment and in a phospholipid bilayer. The simulations show surface association of the peptide with membranes in agreement with solid state NMR data and formation of β-turns in agreement with solution NMR.

 Please wait while we load your content...
Please wait while we load your content...