Very weak interactions: structures, energies and bonding in the tetramers and pentamers of hydrogen sulfide†
Abstract
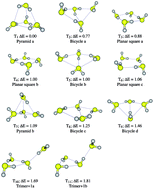
Potential energy surfaces (PESs) for the hydrogen sulfide tetramers and pentamers are shown to be very complex. 11 and 15 different isomers were located on the MP2/6-311++G(d,p) PES of (H2S)4 and (H2S)5 respectively. CCSD(T) energy calculations on the MP2 optimized geometries suggest that all tetramers are within 2.0 kcal mol−1 of the lowest energy structure, while for the pentamers, all structures are found in a 3.5 kcal mol−1 range. To the best of our knowledge, we report and analyze here for the first time in the scientific literature, a newly found type of very weakly stabilizing intermolecular H2S⋯SH2 interaction. In conjunction with traditional H2S⋯H–S–H hydrogen bonds, these previously unreported H2S⋯SH2 intermolecular contacts dictate cluster structures and energies. Our results reveal a very complicated scenario, where a number of different tetramers and pentamers are very close in energy, rendering impossible the unequivocal identification of the global minimum in each case, and as a consequence, suggesting that the properties of these systems would have contributions from many different structures.

 Please wait while we load your content...
Please wait while we load your content...