Synthesis and photocatalytic performance of quasi-fibrous ZnO
Abstract
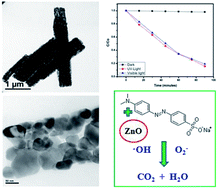
The combustion of oxidizer zinc nitrate and fuel oxalic acid results in quasi-fibrous zinc oxide. The processing parameters including oxidizer to fuel ratio, time and temperature were optimized for the resultant crystal structure and morphology. Pure hexagonal phase formation does not depend on the fuel ratio, but a stoichiometric ratio of oxidizer to fuel at 450 °C and 30 min results in highly crystalline ZnO with 3 μm length and 0.5 μm width. This quasi-fiber originates from partial fusion of near spherical, ∼60 nm particles during the rapid rate of reaction in the combustion process. Transmission electron microscopic analysis confirms the anisotropic primary particle orientation and pore distribution within the developed quasi-fibrous particles. The degradation of methyl orange was assessed by degrading the dye in the presence of the synthesized ZnO (2.95 eV) under both UV and visible light. Quasi-fibrous zinc oxide exhibits effective photocatalytic efficiency under visible light irradiation.

 Please wait while we load your content...
Please wait while we load your content...