Diazo group as a new chemical reporter for bioorthogonal labelling of biomolecules†
Abstract
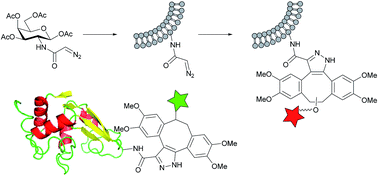
Diazoacetyl groups have been shown to undergo spontaneous cycloaddition reactions with strained alkenes and alkynes. The rates of reaction are similar to the equivalent reactions of azide groups. This allows diazo groups to be used as bioorthogonal reporter groups. This is demonstrated by fluorescent labelling of a diazoacetylated protein and of cell-surface glycans via metabolic incorporation of a diazoacetylated sugar.

 Please wait while we load your content...
Please wait while we load your content...