Transition metal and base free synthesis of 2-aryl-2-oxazolines from aldehydes and β-amino alcohols catalysed by potassium iodide†
Abstract
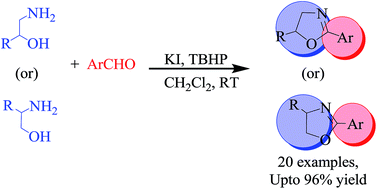
Synthesis of 2-aryl-2-oxazolines from β-amino alcohols and aldehydes was achieved in good to excellent yield by employing a potassium iodide (KI)–tert-butyl hydroperoxide (TBHP) catalytic system. This protocol is very mild, metal and base free and can be performed under ambient reaction conditions. This oxidative cyclization strategy was further extended for the synthesis of optically active 2-oxazolines, which can act as very useful chiral auxiliaries and as ligands.

 Please wait while we load your content...
Please wait while we load your content...