Improving the design of the agarose spot assay for eukaryotic cell chemotaxis
Abstract
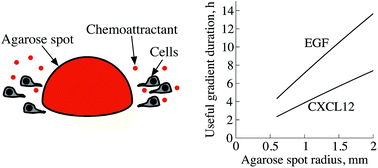
Migration of cells along gradients of effector molecules, i.e., chemotaxis, is necessary in immune response and is involved in development and cancer metastasis. The experimental assessment of chemotaxis thus is of high interest. The agarose spot assay is a simple tissue culture system used to analyze chemotaxis. Although direction sensing requires gradients to be sufficiently steep, how the chemical gradients developed in this assay change over time, and thus, under what conditions chemotaxis is plausible, has not yet been determined. Here, we use numerical solution of the diffusion equation to determine the chemoattractant gradient produced in the assay. Our analysis shows that, for the usual spot size, the lifetime of the assay is optimized if the chemoattractant concentration in the spot is initially 30 times the dissociation constant of the chemoattractant-receptor bond. This result holds regardless of the properties of the chemoattractant. With this initial concentration, the chemoattractant gradient falls to the minimum threshold for directional sensing at the same time that the concentration drops to the optimal level for detecting gradient direction. If a higher initial chemoattractant concentration is used, the useful lifetime of the assay is likely to be shortened because receptor saturation may decrease the cells' sensitivity to the gradient; lower initial concentrations would result in too little chemoattractant for the cells to detect. Moreover, chemoattractants with higher diffusion coefficients would sustain gradients for less time. Based on previous measurements of the diffusion coefficients of the chemoattractants EGF and CXCL12, we estimate that the assay will produce gradients that cells can sense for a duration of 10 h for EGF and 5 h for CXCL12. These gradient durations are comparable to what can be achieved with the Boyden chamber assay. The analysis presented in this work facilitates determination of suitable parameters for the assay, and can be used to assess whether observed cell motility is likely due to chemotaxis or chemokinesis.


 Please wait while we load your content...
Please wait while we load your content...