A computational study on the mechanism and kinetics of the reaction between CH3CH2S and OH†
Abstract
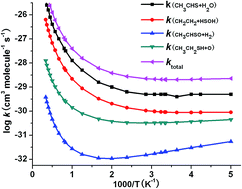
The reaction mechanism of CH3CH2S with OH radicals is studied at the CBS-QB3 level of theory. Five substitution processes and eleven addition–elimination channels are identified for the title reaction. The calculated results indicate that addition–elimination channels CH3CHS + H2O, CH2CH2 + HSOH, CH3CHSO + H2 and CH3CH2SH + O are dominant. Other channels may be negligible due to the high barrier heights. Rate constants and branching ratios are estimated by means of the conventional transition state theory with zero curvature tunnelling over the temperature range of 200–3000 K. The calculation shows that the overall rate constant in the temperature of 200–3000 K is mainly dependent on the channels CH3CHS + H2O, CH2CH2 + HSOH and CH3CH2SH + O. The three-parameter expression for the total rate constant is fitted to be ktotal = 7.42 × 10−21T2.63 exp(−772.43/T) cm3 molecule−1 s−1 between 200–3000 K.

 Please wait while we load your content...
Please wait while we load your content...