Synthesis, crystal structure, DNA interaction and in vitro anticancer activity of a Cu(ii) complex of purpurin: dual poison for human DNA topoisomerase I and II†
Abstract
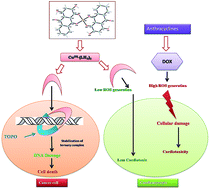
Although generation of reactive oxygen species (ROS) by anthracycline anticancer drugs is essential for anti-tumor activity, they make these drugs cardiotoxic. Metal–anthracyclines that generate relatively fewer ROS are however, effective antitumor agents. Purpurin (LH3), a hydroxy-9,10-anthraquinone, closely resembles doxorubicin, an established anthracycline drug. This molecule was chosen to study the extent to which simpler analogues are effective. A Cu(II) complex of LH3 [Cu(II)–(LH2)2] was synthesized to mimic the metal–anthracycline complexes. The crystal structure of [Cu(II)–(LH2)2] was determined by Rietveld refinement of PXRD data using an appropriate structural model developed on the basis of spectroscopic data. This is the first report on the crystal structure of any hydroxy-9,10-anthraquinone with a 3d-transition metal ion. The bond lengths and bond angles obtained by structural refinement corroborate those calculated by the DFT method. DNA binding of the complex was slightly better than purpurin. However, more importantly, unlike purpurin, binding constant values did not decrease with increasing pH of the medium. DNA relaxation assays show Cu(II)–(LH2)2 as a novel potent dual inhibitor of human DNA topoisomerase I and topoisomerase II enzymes. Cu(II)–(LH2)2 stabilizes covalent topoisomerase–DNA adducts both in vitro and within cancer cells. The cleavage assay keeps the complex well ahead of LH3 with regard to efficacy. These results paralleled those of cell growth inhibition and showed that the complex was more effective in killing ALL MOLT-4 cells than LH3, suggesting it targets topoisomerase enzymes within cells. The NADH dehydrogenase assay revealed further that the generation of superoxide was less in the case of the complex as compared to LH3.

 Please wait while we load your content...
Please wait while we load your content...