A simple and efficient mechanochemical route for the synthesis of 2-aryl benzothiazoles and substituted benzimidazoles†
Abstract
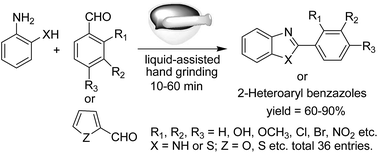
An efficient and versatile mechanochemical route to 2-aryl benzothiazoles and both 2-substituted and 1,2-disubstituted benzimidazole derivatives has been developed via a simple mortar–pestle grinding method. The mechanochemical agitation was found to be sufficient for smooth condensation between a variety of aromatic aldehydes and o-aminothiophenol/o-phenylenediamine followed by cyclization leading to the formation of the corresponding 1,3-benzazoles. The salient features of this new protocol are catalyst-free reaction, cleaner reaction profiles, absence of a work-up step, high yields, and short reaction times.

 Please wait while we load your content...
Please wait while we load your content...