Molecular dynamics study of Congo red interaction with carbon nanotubes
Abstract
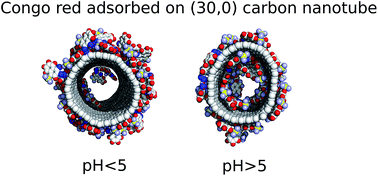
This work deals with molecular dynamics simulations of Congo red (CR) interaction with carbon nanotubes (CNT). We studied several combinations of systems parameters in order to assess how the nanotube diameter and Congo red density affect the structure and stability of CNT–CR conjugates at various pH conditions. We found that, at the considered conditions, the CR binds strongly to the CNT surfaces and the CNT–CR conjugates are thermodynamically stable according to the determined values of the free energies. Adsorption on wider nanotubes is stronger than on the narrow ones and larger densities of CR on the CNT surfaces lead to weakening of binding energy per single CR molecule. Changes of pH, that is varying concentration of protonated and deprotonated forms of CR, lead to significant changes in binding energies as well as to qualitative changes of the structure of the adsorbed CR. It was found that at pH > 5.5 the CR molecules readily occupy inner cavities of the nanotubes. Upon lowering pH the occupation of the inner space of CNTs is strongly reduced and the preferred configuration is formation of a densely packed CR layer on the sidewalls of the CNT. This effect can potentially be utilized in pH controlled corking/uncorking of carbon nanotubes in water solutions.

 Please wait while we load your content...
Please wait while we load your content...