Combined heterogeneous bio- and chemo-catalysis for dynamic kinetic resolution of (rac)-benzoin†
Abstract
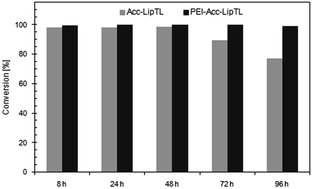
Dynamic kinetic resolution (DKR) of racemic starting material is a promising route to optically pure (S)-benzoin (2-hydroxy-1,2-di(phenyl)ethanone) and various symmetrical and unsymmetrical derivatives thereof. Here, this route was advanced towards technical scale synthesis using the basic (rac)-benzoin as model system. The reaction employed stereoselective transesterification of (S)-benzoin with lipase TL® from Pseudomonas stutzeri and racemization of (R)-benzoin with Metal-TUD-1, a metal-associated acidic meso-porous silicate, in pure organic solvent. Enzyme performance was improved by immobilization on Accurel MP1001 (yielding Acc-LipTL), and Zr-TUD-1 (Si/Zr = 25) was identified as most effective racemization catalyst. Compatibility in solvent and temperature dependency enabled performance in only one pot. DKR in toluene at 50 °C yielded conversions above 98% and an ee of >97% in only five hours. Stability of Acc-LipTL was further improved with polyethylene imine and the reaction system was then reused in five cycles, retaining a conversion of >99% and a product-ee of >98%. On a semi-preparative scale, the isolated yield of enantiopure (S)-benzoin butyrate was >98%. Thus, the system provides a good basis for synthesis of enantiopure benzoin, and potentially a broader range of aromatic α-hydroxy ketones.

 Please wait while we load your content...
Please wait while we load your content...