Ball milling for the quantitative and specific solvent-free Knoevenagel condensation + Michael addition cascade in the synthesis of various 2-amino-4-aryl-3-cyano-4H-chromenes without heating†
Abstract
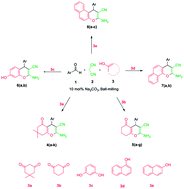
Ball milling is used as a facile, efficient, cheap, and environmentally friendly procedure for the solvent-free three-component reaction of aromatic aldehydes with malononitrile and dimedone, or 1,3-cyclohexanedione, resorcinol, and α- and β-naphthol. The reactions proceed quantitatively at room temperature by milling stoichiometric mixtures of the reagents in the presence of 10 mol% Na2CO3. This method offers the advantages of short reaction times, low cost, quantitative yields and simple work-up with no need of any organic solvent. It is thus enviro-economic without producing dangerous wastes. The orientation selectivity with β-naphthol and resorcinol is discussed. Existing structural misattributions are pointed out and clarified by 1H NMR spin coupling analysis.

 Please wait while we load your content...
Please wait while we load your content...