Acetamidine–palladium complex immobilized on γ-Fe2O3 nanoparticles: a novel magnetically separable catalyst for Heck and Suzuki coupling reactions
Abstract
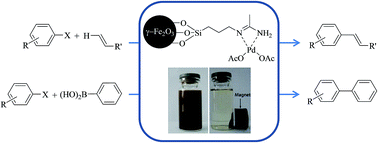
A new palladium–Schiff base complex immobilized on iron oxide nanoparticles (γ-Fe2O3-acetamidine-Pd) was synthesized via the reaction of amino-functionalized γ-Fe2O3 with acetamide followed by the reaction with palladium acetate. Characterization of γ-Fe2O3-acetamidine-Pd was carried out by various techniques such as XRD, SEM, HRTEM, FT-IR, TGA, ICP, XPS and elemental analysis. γ-Fe2O3-acetamidine-Pd was successfully applied as a magnetically recyclable catalyst in Heck and Suzuki coupling reactions. By these protocols, aryl halides were coupled with olefins (Heck coupling reaction) and phenylboronic acid (Suzuki coupling reaction) to afford the corresponding products in moderate to high yields. Furthermore, the synthesized catalyst was separated easily by using an external magnet and recycled for five runs without appreciable loss of its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...