Dissociative electron attachment to the complexation ligands hexafluoroacetylacetone, trifluoroacetylacetone and acetylacetone; a comparative experimental and theoretical study
Abstract
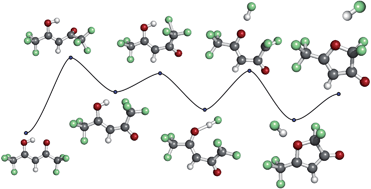
The β-diketones acetylacetone (AAc), trifluoroacetylacetone (TFAAc), and hexafluoroacetylacetone (HFAAc), are commonly used as ligands for metal complexes in applications where relatively high stability and vapour pressure is required. While fluorination of the native AAc generally increases both stability and vapour pressure of the respective metal complexes it also alters the electronic structure, and thereby the susceptibility to bond cleavage by low energy electrons. Here we present a detailed comparative study on dissociative electron attachment (DEA) to the isolated ligands AAc, TFAAc and HFAAc in the energy range from 0–15 eV. While single bond ruptures at fairly high energies dominate in DEA to the native AAc, extensive fragmentation, new bond formation and rearrangement is observed from the fluorinated β-diketones. These reactions have high cross sections at 0 eV, where they are often associated with stabilisation through H⋯F hydrogen bond formation and HF loss. From HFAAc considerable contributions are also observed at about 1 and 3 eV. Through comparison of the three compounds and quantum chemical calculations of the threshold energies for individual processes we are able to offer a plausible picture of the reaction dynamics behind the bulk of these channels. Finally, for the most dominating reaction channel, i.e., the loss of HF from HFAAc, we calculate the minimum energy path by using the nudged elastic band method.

 Please wait while we load your content...
Please wait while we load your content...