Understanding the stability, bonding nature and chemical reactivity of 3d-substituted heterofullerenes C58TM (TM = Sc–Zn) from DFT studies†
Abstract
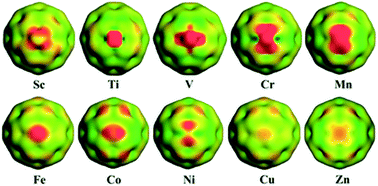
The stabilities, bonding nature and reactivity of C58TM (TM = Sc–Zn) heterofullerenes are investigated using DFT methods. The [6:6] structure is more stable than the [5:6] isomer for all of the studied heterofullerenes. Among the title heterofullerenes, C58Zn has the largest heat of formation, and shows low thermal stability due to a great expansion deformation of the C58 cage. For open-shell heterofullerenes, the spin densities are localized on the TM atoms, except for C58Sc and C58Cu in which the spin densities are delocalized over the C58 cage. The QTAIM analyses indicate that the TM–C bonds show a partial covalent characteristic. The degree of covalence slightly increases from left to right across the 3d-block of the periodic table. The chemical reactivity of the studied heterofullerenes can be tuned by substituted TM atoms, deduced from frontier molecular orbitals and Fukui function analyses. The Fukui function also shows that the earlier TM-substituted species, C58Ti, C58V, C58Cr and C58Mn, are more active than other heterofullerenes. The adsorption of ethylene molecules on heterofullerenes is investigated to probe the chemical reactivity of the studied heterofullerenes; the fact that earlier TM atoms show greater reactivity than later TM atoms is in excellent agreement with Fukui function predictions. Moreover, the adsorption energies (Eads) of earlier TM-substituted heterofullerenes are comparable to that of the previously reported C58Ir(C2H4) complex.

 Please wait while we load your content...
Please wait while we load your content...