Stable anatase TiO2 formed by calcination of rice-like titania nanorod at 800 °C exhibits high photocatalytic activity†
Abstract
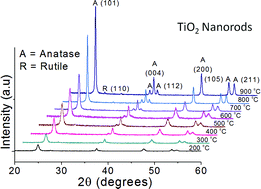
This paper demonstrates the complete retention (>98%) of anatase TiO2 crystalline phase after high temperature (800 °C) thermal treatment of rice-like TiO2 nanorods (length = 81–134 nm, diameter = 8–13 nm) relative to 100% conversion of the rutile phase after calcination of P25-TiO2 under similar conditions. The existence of the anatase phase at >800 °C was further confirmed by the presence of characteristic vibrational bands (144, 395, 513 and 639 cm−1) in the Raman spectra. It was found that TiO2 nanorods undergo fragmentation to a highly crystalline irregular morphology (60–70 nm), nanopolygons (91–110 nm) and smaller rod-shaped particles (length = 60–110 nm and diameter = 7–12 nm), accompanied by a gradual increase in their crystallite size (from 16 to 40 nm) and decrease in surface area (from 79 to 31 m2 g−1) with increased calcination temperatures from 200 to 900 °C. This TiO2 anatase phase displayed enhanced photocatalytic oxidation rate (∼2–11 times higher than rutile TiO2) for methyl parathion (a neurotoxic pesticide) degradation to various intermediate products and ultimately to CO2, whereas 1.0 wt% Au–TiO2 significantly improved the photoactivity.

 Please wait while we load your content...
Please wait while we load your content...