An expedient route to heterocycles through α-arylation of ketones and arylamides by microwave induced thermal SRN1 reactions†
Abstract
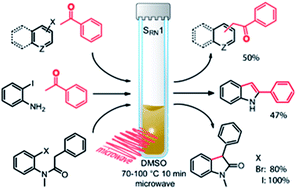
Microwave irradiation promotes a quick aromatic nucleophilic substitution by a thermally induced electron transfer process to form new C–C bonds by the coupling of aryl radicals and enolate nucleophiles. Diverse 2-aryl-1-phenylethanones can be prepared by the direct α-arylation of acetophenone with different haloarenes. The ketone enolate anion is generated by deprotonation with tBuOK in DMSO and the reaction is carried out in a closed microwave vessel at 70–100 °C for 10 min. This simple procedure also allows the synthesis of deoxybenzoin and indole heterocycle derivatives by inter- or intra-molecular ring closure reactions, with moderate to excellent substitution yields.

 Please wait while we load your content...
Please wait while we load your content...