Electron induced chemistry of disilane
Abstract
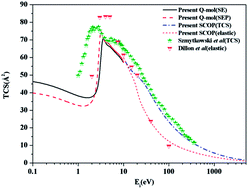
Theoretical study of electron impact scattering by disilane molecule is reported in this article. Total, elastic, excitation and differential cross sections were computed at low incident energies using the R-matrix method through QUANTEMOL-N. The total cross section calculation was extended to higher energies using spherical complex optical potential formalism. The smooth transition at the overlap of two formalisms around the ionization threshold of the target has helped to predict cross sections over a wide energy range from 0.1 eV to 5 keV. The resonance position predicted by the present static exchange and static exchange plus polarization models at 3.3 and 3.0 eV respectively agrees quite well with previous theoretical and experimental results. The inclusion of polarization effects in the calculation has considerably improved the position of the resonance from the previous static exchange calculation. In general the results obtained for total, elastic and differential cross sections show reasonable agreement with the experiment. The excitation cross section of disilane from ground state to various excited states is reported for the first time.

 Please wait while we load your content...
Please wait while we load your content...