Abinitio assessment of graphene nanoribbons reactivity for molecule adsorption and conductance modulation: nitrogen dioxide nanosensor
Abstract
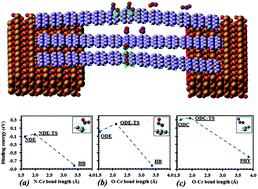
Adsorption of functional groups on graphene nanoribbons has fundamental impacts on their applications in areas as diverge as energy storage, nanoelectronics, drug delivery, and sensors. To reveal adsorption geometries, energy barriers, and room temperature rate constants for assessing reaction kinetics, we study interactions of NO2 molecules with ultra-narrow (∼1 nm) hydrogen terminated armchair graphene nanoribbons (AGNRs), using first principles. We show that formation of hydrogen bonded NO2 at the edge and physisorbed NO2 at the center are processes without barriers, whereas chemisorption at center or edge are activated processes. Nonbonding and weak sp3 hybridization at the edge of AGNR are shown to be more favorable than center adsorptions, revealing increased reactivity compared to graphene. Resultant modulations of quantum transport are calculated for sensing extremely low gas concentrations. Detectable current decrease is predicted for two hydrogen-bonded or chemisorbed molecules. We discuss possible measures to enhance sensitivity of GNRs for detecting extremely low concentrations of nitrogen dioxide and similar molecules.

 Please wait while we load your content...
Please wait while we load your content...