Synthesis of Fe-doped octahedral Pt3Ni nanocrystals with high electro-catalytic activity and stability towards oxygen reduction reaction†
Abstract
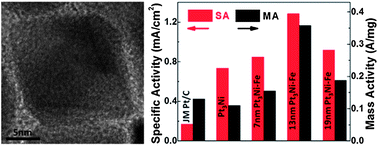
Octahedral Pt3Ni nanocrystals doped with Fe (Pt3Ni-Fe) are synthesized through solvothermal methods in N,N-dimethylformamide solvent at 210 °C. By manipulating the amount of combinational capping agents, the size of the octahedral Pt3Ni-Fe nanocrystals can be controlled between 7 and 19 nm. It is found that one of the capping agents, n-octadecylphosphonic acid, is critical in obtaining the octahedral morphology. A volcano-shaped relation between oxygen reduction reaction (ORR) activity and nanocrystal size is determined for the carbon-supported octahedral Pt3Ni-Fe, with the highest ORR activity observed from the 13 nm nanocrystal. It is also found that Pt3Ni-Fe electro-catalysts show enhanced stability. This work demonstrates that Fe-doping can enhance the electro-catalytic performance of octahedral Pt3Ni.

 Please wait while we load your content...
Please wait while we load your content...