Fe/S-catalyzed decarboxylative redox condensation of arylacetic acids with nitroarenes†
Abstract
Fe/S clusters generated in situ from simple iron salts and sulfur S8 were found to be highly efficient to catalyze the decarboxylative redox condensation of arylacetic acids with nitroarenes in the presence of N-methylpiperidine as a basic additive. A wide range of aza-heterocycles was obtained in an atom-, step-, and redox-economical manner with water and carbon dioxide as the only by-products.
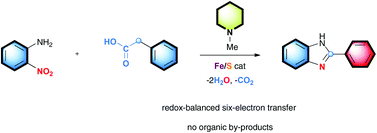

 Please wait while we load your content...
Please wait while we load your content...