Synthesis of 1,4-benzodiazepinones and 1,4-benzoxazepinones via palladium-catalyzed amino and oxyacetoxylation†‡
Abstract
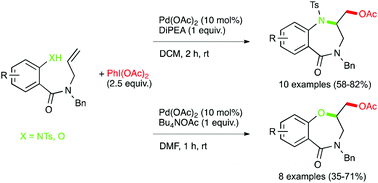
Palladium-catalyzed amino and oxyacetoxylation have been developed to furnish 1,4-benzodiazepinones and 1,4-benzoxazepinones through the diheterofunctionalization of alkenes. This study demonstrates the ability of this new methodology to allow 7-exo ring-closure.
- This article is part of the themed collection: In Celebration of Max Malacria’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...