Carbopalladation of bromoene-alkynylsilanes: mechanistic insights and synthesis of fused-ring bicyclic silanes and phenols†
Abstract
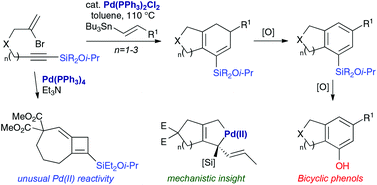
The palladium-catalyzed cascade cyclization of silylated bromoenynes and alkenylstannanes provides a straightforward route to a range of bicyclic silylated cyclohexadienes. Mechanistic insights into aspects of carbopalladation and unusual palladium-mediated isomerizations have been obtained through the detection of reaction intermediates, the isolation of byproducts, and reaction monitoring by VT NMR spectroscopy. The utility of the bicyclic products is illustrated through oxidation to bicyclic enones and phenols.

 Please wait while we load your content...
Please wait while we load your content...