Stereoselective intramolecular cyclopropanation of α-diazoacetates via Co(ii)-based metalloradical catalysis†
Abstract
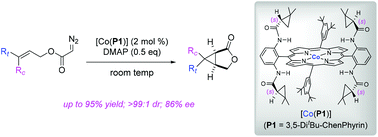
Co(II) complexes of D2-symmetric chiral porphyrins have been proven to be effective metalloradical catalysts for the asymmetric intramolecular cyclopropanation of allyl α-diazoacetates. 4-(Dimethylamino)pyridine (DMAP), through a positive trans effect, plays an important role in the enhancement of the asymmetric induction for the intramolecular cyclopropanation process. This metalloradical catalytic system is suitable for cyclopropanation of allyl α-diazoacetates with varied functional groups and substitution patterns, producing bicyclic products with complete diastereocontrol and good enantiocontrol.

 Please wait while we load your content...
Please wait while we load your content...