Modulating the copper–sulfur interaction in type 1 blue copper azurin by replacing Cys112 with nonproteinogenic homocysteine†
Abstract
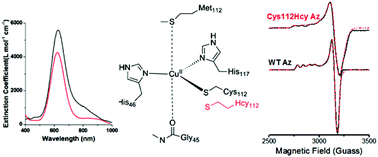
The Cu–SCys interaction is known to play a dominant role in defining the type 1 (T1) blue copper center with respect to both its electronic structure and electron transfer function. Despite this importance, its role has yet to be probed by mutagenesis studies without a dramatic change in its T1 copper character. We report herein replacement of the conserved Cys112 in azurin with the nonproteinogenic amino acid homocysteine. Based on electronic absorption, electron paramagnetic resonance, and extended X-ray absorption fine structural spectroscopic studies, this variant displays typical type 1 copper site features. Surprisingly, instead of increasing the strength of the Cu–sulfur interaction by the introduction of the extra methylene group, the Cys112Hcy azurin showed a decrease in the covalent interaction between SHcy and Cu(II) when compared with the WT SCys–Cu(II) interaction. This is likely due to geometric adjustment of the center that resulted in the copper ion moving out of the trigonal plane defined by two histidines and one Hcy and closer to Met121. These structural changes resulted in an increase in reduction potential by 35 mV, consistent with lower Cu–S covalency. These results suggest that the Cu–SCys interaction is close to being optimal in native blue copper protein. It also demonstrates the power of using nonproteinogenic amino acids in addressing important issues in bioinorganic chemistry.
- This article is part of the themed collection: HOT articles in Inorganic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...