Thermally cleavable imine base/isocyanate adducts and oligomers suitable as initiators for radical homo- and copolymerization†
Abstract
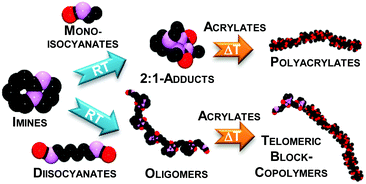
The addition of isocyanates to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds of imines gave triazinedione heterocycle structures; their thermal properties are reported. Mono-isocyanates were used to form 2 : 1 adducts with the imine bases 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) and 2-tert-butyl-1,1,3,3-tetramethylguanidine (tBuTMG). A 2 : 1 stoichiometry of the adducts was proven by NMR and IR spectroscopy and single crystal X-ray diffraction; certain cleavage temperatures (70 and 160 °C) were measured. Thermal analysis (TG-MS) of adducts indicated the release of free isocyanate during adduct cleavage. Furthermore, a new class of step-growth oligomers (MN = 750–7000 g mol−1) composed of multi-functional isocyanates and these imine bases was introduced. Their systematic spectroscopic and thermal analysis was shown, revealing the similarity in their chemical properties to the 2 : 1 adducts. Radical homo- and copolymerization of acrylates was initiated by the meta-stable adducts and oligomers of this work; the generation of novel telomeric block-copolymer architectures composed of polyacrylate and oligourea building blocks was demonstrated.
N double bonds of imines gave triazinedione heterocycle structures; their thermal properties are reported. Mono-isocyanates were used to form 2 : 1 adducts with the imine bases 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1,5-diazabicyclo[4.3.0]non-5-ene (DBN) and 2-tert-butyl-1,1,3,3-tetramethylguanidine (tBuTMG). A 2 : 1 stoichiometry of the adducts was proven by NMR and IR spectroscopy and single crystal X-ray diffraction; certain cleavage temperatures (70 and 160 °C) were measured. Thermal analysis (TG-MS) of adducts indicated the release of free isocyanate during adduct cleavage. Furthermore, a new class of step-growth oligomers (MN = 750–7000 g mol−1) composed of multi-functional isocyanates and these imine bases was introduced. Their systematic spectroscopic and thermal analysis was shown, revealing the similarity in their chemical properties to the 2 : 1 adducts. Radical homo- and copolymerization of acrylates was initiated by the meta-stable adducts and oligomers of this work; the generation of novel telomeric block-copolymer architectures composed of polyacrylate and oligourea building blocks was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...