Synthesis of fluorinated alkoxyamines and alkoxyamine-initiated nitroxide-mediated precipitation polymerizations of styrene in supercritical carbon dioxide†
Abstract
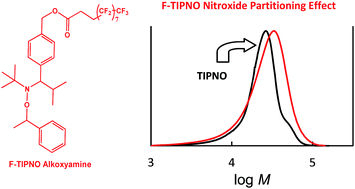
TIPNO (2,2,5-trimethyl-4-phenyl-3-azahexane-3-nitroxide)-alkoxyamine was found to give reasonably controlled/living nitroxide-mediated (NMP) precipitation polymerizations of styrene in supercritical carbon dioxide (scCO2). In contrast under the same conditions, the analogous SG1 (N-tert-butyl-N-(1-diethylphosphono-2,2-dimethylpropyl)nitroxide)-alkoxyamine gave higher rates of polymerization and inferior controlled/living character. The circumvention of the requirement for excess free [nitroxide]0 allowed the study of nitroxide partitioning effects in scCO2 for three newly synthesized fluorinated alkoxyamines. Two alkoxyamines dissociated into scCO2-philic fluorinated TIPNO-nitroxide derivatives, while another contains a similar sized fluorinated “foot”. Despite the increased steric bulk about the N–O bond for the novel fluorinated alkoxyamines, all polymerizations proceeded at a similar rate and level of control to the TIPNO system in solution (toluene). PREDICI simulations for the styrene/TIPNO system are used to support extensive partitioning effects observed in scCO2 for the fluorinated alkoxyamines.

 Please wait while we load your content...
Please wait while we load your content...