One-step synthesis of polylactide macrocycles from sparteine-initiated ROP†
Abstract
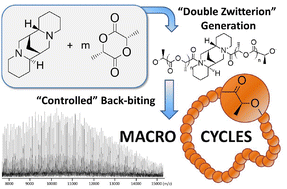
The ring-opening polymerization (ROP) of L-lactide in dichloromethane with (+)-sparteine is described. Under aprotic conditions, (+)-sparteine leads to controlled cyclic polyesters mainly obtained by a backbiting process from the in situ generated tertiary amine-containing symmetrical binary zwitterion.

 Please wait while we load your content...
Please wait while we load your content...